Product Name:Hawthorn Leaf Extract
Latin Name:Crataegus Pinnatifida Bge
CAS No:3681-93-4
Plant Part Used:Leaf
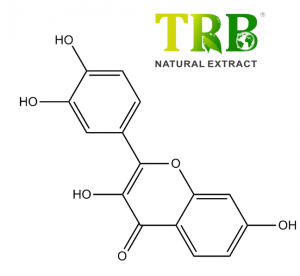
Assay:Vitexin-2-0-rhamnoside≧1.8% by HPLC;
Colour: Red brown powder with characteristic odor and taste
GMO Status:GMO Free
Packing: in 25kgs fiber drums
Storage:Keep container unopened in cool, dry place,Keep away from strong light
Shelf Life:24 months from date of production
Hawthorn Leaf Extract Vitexin-2-O-Rhamnoside ≥1.8% by HPLC
Premium Standardized Extract for Cardiovascular & Antioxidant Support
Product Overview
Our Hawthorn Leaf Extract is a scientifically formulated dietary supplement ingredient derived from Crataegus pinnatifida leaves, standardized to contain ≥1.8% Vitexin-2-O-Rhamnoside as verified by High-Performance Liquid Chromatography (HPLC) . Native to Asia, Europe, and North America, hawthorn has been used for centuries in traditional medicine to support cardiovascular health and digestion . This extract combines ancient wisdom with modern quality control to deliver a potent, consistent product tailored for global markets.
Key Features
- Standardized Potency:
- ≥1.8% Vitexin-2-O-Rhamnoside, a bioactive flavonoid with proven antioxidant and anti-inflammatory properties .
- Rigorous HPLC testing ensures batch-to-batch consistency and purity .
- Clinically Supported Benefits:
- Cardiovascular Support: Promotes healthy blood pressure, improves coronary circulation, and reduces cholesterol levels .
- Antioxidant Protection: Neutralizes free radicals, mitigating oxidative stress and inflammation .
- Digestive Health: Enhances gut barrier function and alleviates food stagnation .
- Quality Assurance:
- Manufactured in GMP-certified facilities with ISO compliance .
- Third-party validated by FDA-registered agencies for identity, potency, and safety .
Applications
- Dietary Supplements: Ideal for heart health formulas, antioxidant blends, and digestive aids.
- Functional Foods: Incorporate into beverages, powders, or capsules for added health benefits.
- Pharmaceuticals: Used in formulations targeting hypertension, arrhythmia, and metabolic syndrome .
Technical Specifications
- Active Ingredient: Vitexin-2-O-Rhamnoside ≥1.8% (HPLC).
- Botanical Source: Crataegus pinnatifida leaf.
- Appearance: Fine brown powder.
- Certifications: Non-GMO, Allergen-Free, Heavy Metal Tested.
- Packaging: 1 kg to 25 kg sealed drums with dual plastic liners for optimal freshness .
Why Choose Our Extract?
- Keywords: “Hawthorn Leaf Extract,” “Vitexin-2-O-Rhamnoside,” “HPLC-Verified,” “Cardiovascular Support”
- Localized Compliance: Labeling and documentation meet EU and US regulatory standards.
- Custom Solutions: Available in bulk quantities and custom formulations (e.g., combined with polysaccharides or other flavonoids) .
Dosage & Safety
- Recommended Dose: 250–500 mg daily, depending on application .
- Safety Profile: Generally recognized as safe (GRAS) with no significant side effects at recommended doses