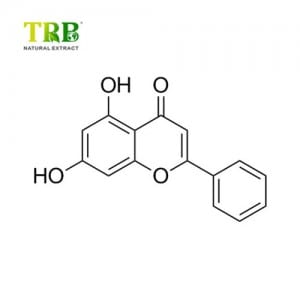
Product Name:Maitake Mushroom Extract/Grifola Frondosa extract
Latin Name:Lentinus Edodes(Berk.)Sing
CAS No:37339-90-5
Plant Part Used:Fruit
Assay:Polysaccharides 0.50%~50.0% by UV
Colour:Yellowish brown powder with characteristic odor and taste
GMO Status:GMO Free
Packing: in 25kgs fiber drums
Storage:Keep container unopened in cool, dry place,Keep away from strong light
Shelf Life:24 months from date of production
Maitake Mushroom Extract: The Natural Immune Booster and Wellness Enhancer
Looking for a natural way to boost your immune system, support healthy blood sugar levels, and promote overall well-being? Maitake Mushroom Extract is a powerful herbal supplement derived from the Grifola frondosa mushroom, a medicinal fungus native to Japan and North America. Known as the “Dancing Mushroom,” Maitake Mushroom Extract is packed with beta-glucans, polysaccharides, and antioxidants, making it a trusted choice for individuals seeking natural solutions for immune support, metabolic health, and overall vitality. Whether you’re looking to strengthen your immune system, regulate blood sugar, or simply enhance your daily wellness routine, this extract offers a science-backed, plant-based option.
What is Maitake Mushroom Extract?
Maitake Mushroom Extract comes from the fruiting body of the Grifola frondosa mushroom, which is rich in bioactive compounds like beta-glucans, polysaccharides, and ergosterols. These compounds are responsible for its immune-boosting, anti-inflammatory, and blood sugar-regulating properties. Traditionally used to support overall health and vitality, Maitake Mushroom Extract is now backed by modern research for its wide range of benefits.
Key Benefits of Maitake Mushroom Extract
- Boosts Immune Function
Maitake Mushroom Extract is widely recognized for its ability to enhance the immune system, helping your body fight off infections and illnesses more effectively. - Supports Healthy Blood Sugar Levels
The extract helps improve insulin sensitivity and regulate blood sugar levels, making it beneficial for individuals with metabolic concerns. - Rich in Antioxidants
Packed with polysaccharides and other antioxidants, Maitake Mushroom Extract helps neutralize free radicals, protecting cells from oxidative stress and supporting healthy aging. - Promotes Heart Health
Maitake Mushroom Extract helps maintain healthy cholesterol levels and supports proper blood circulation, contributing to better cardiovascular health. - Anti-Inflammatory Properties
The bioactive compounds in Maitake Mushroom Extract help reduce inflammation, making it beneficial for individuals with joint pain, arthritis, or other inflammatory conditions. - Supports Weight Management
The extract helps promote healthy metabolism and fat burning, making it a valuable addition to a weight management plan. - Enhances Overall Vitality
By supporting immune function and metabolic health, Maitake Mushroom Extract helps boost energy levels and promote overall well-being.
Why Choose Our Maitake Mushroom Extract?
- Premium Quality: Our extract is sourced from organically grown Maitake mushrooms, ensuring the highest purity and potency.
- Scientifically Formulated: We use advanced extraction methods to preserve the bioactive compounds, delivering maximum benefits.
- Third-Party Tested: Every batch is rigorously tested for quality, safety, and efficacy.
- Eco-Friendly Packaging: We are committed to sustainability, using recyclable materials for our products.
How to Use Maitake Mushroom Extract
Our Maitake Mushroom Extract is available in convenient forms, including capsules, liquid tinctures, and powders. For optimal results, follow the recommended dosage on the product label or consult with a healthcare professional.
Customer Reviews
“Maitake Mushroom Extract has been a game-changer for my immune system and energy levels. I feel more resilient and active!” – Emily R.
“This product has helped regulate my blood sugar and improve my overall well-being. Highly recommend it to anyone looking for a natural health boost.” – Michael T.
Discover the Benefits Today
Experience the transformative power of Maitake Mushroom Extract and take the first step toward better immune support, metabolic health, and overall wellness. Visit our website to learn more and place your order. Don’t forget to subscribe to our newsletter for exclusive offers and health tips!
Description:
Unlock the natural benefits of Maitake Mushroom Extract – a premium supplement for immune support, blood sugar regulation, and overall wellness. Shop now for high-quality, eco-friendly products!
Maitake Mushroom Extract, immune booster, blood sugar support, antioxidants, heart health, anti-inflammatory, weight management, natural supplements, eco-friendly health products